Introduction
DNA length polymorphisms are difficult to diagnose due to their repetitive or variable elements. Within the FLT3 gene, internal tandem duplications (ITDs) range from <30 bp to >200 bp in length and are associated with poor prognosis in acute myeloid leukemia (AML). To address these challenges, we developed digital polymerase chain reaction (dPCR) followed by high-speed atomic force microscopy (HSAFM) as a high-throughput, single-molecule approach for quantifying and sizing length polymorphisms associated with FLT3-ITDs.
Methods
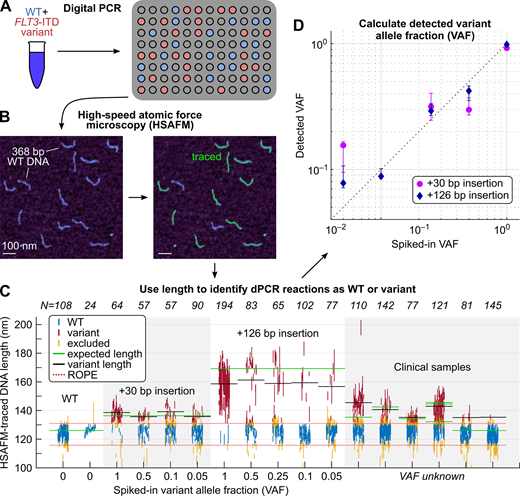
In the first step of our approach, dPCR (Fig. 1A), a mixed sample is diluted and partitioned into micro-reactions that are individually amplified, mitigating amplification bias and producing homogeneous solutions of either wild-type (WT) or FLT3-ITD variant amplicons. We then used HSAFM to directly image individual amplicons and determine their lengths with nanoscale resolution (Fig. 1B). Length distributions were analyzed by Bayesian inference to identify their most likely character-WT or variant-by determining whether or not the 95% most credible range of mean lengths for each micro-reaction fell within a region of practical equivalence (ROPE) to WT length (Fig. 1C). Variant allele frequency (VAF) was then calculated as the proportion of variant-identified micro-reactions (Fig. 1D). We tested (a) synthetic and cell line DNA ranging from 30 bp to 217 bp, with VAF titrated down to 5%, and (b) clinical FLT3-ITD positive and negative samples, and compared dPCR-HSAFM results to those of a widespread clinical assay (Invivoscribe Leukostrat CDx FLT3 Mutation Assay).
Results
dPCR-HSAFM successfully returned the VAFs of all spiked-in FLT3-ITD samples across the admixture range of 5%-100%, and WT-only controls yielded VAFs of 0% (Fig. 1C,D). The four tested clinical samples determined as FLT3-ITD positive by the standard assay returned VAFs of 49%, 16%, 26%, and 61% by dPCR-HSAFM, while the two FLT3-ITD negative clinical samples returned VAFs of 6% and <1% (Fig. 1C). Mean variant length of control and clinical samples measured by dPCR-HSAFM agreed with the known lengths as reported by the Leukostrat assay or given by cell line documentation (Fig. 1C, horizontal lines).
Conclusions
By demonstrating accurate quantification and sizing of FLT3-ITD variants of clinically relevant size and VAF, dPCR-HSAFM supersedes the standard clinical assay, which does not report VAF. Since FLT3-ITD length and VAF are linked to AML outcome and are important for minimal residual disease following therapy, a test that provides both metrics is desirable. Furthermore, the speed, low cost, and flexibility of dPCR-HSAFM to detect long, repetitive insertions make it an attractive alternative to sequencing in the clinical diagnosis of FLT3-ITDs and other length polymorphisms.
Figure 1. dCR-HSAFM technique and results. (A) Digital PCR of a mixed sample yields a homogeneous population of WT (blue) or variant FLT3-ITD (red) amplicons in each micro-reaction. (B) HSAFM images of DNA are traced with custom software to yield lengths of individual DNA molecules. (C) Length is used to classify each dPCR reaction as WT or variant, where each vertical line gives the 95% most credible range of mean length for a dPCR reaction and italicized numbers give the number of dPCR reactions for each sample. The four leftmost clinical samples were identified as FLT3-ITD positive by a standard clinical assay, while the two rightmost clinical samples were FLT3-ITD negative. The assay does not report VAF. (D) Spiked-in VAF v. detected VAF is shown for FLT3-ITD samples with 30 bp and 126 bp insertions, with spiked-in VAF ranging from 5%-100%. Vertical bars give the VAFs for ROPEs ± 10% of the initial ROPE width, which conveys the sensitivity of each VAF to the ROPE width.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal